Fda Approval
Advertisement
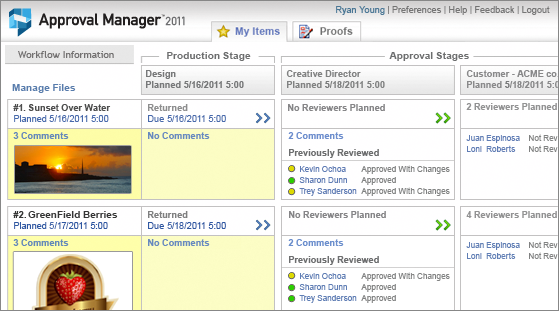
Approval Manager Express v.2011
Approval Manager 2011 Express is the #1 Free Workflow Automation App for Online Proofing & Review.
Advertisement
HEC-FDA v.1.2.5.1
The Flood Damage Reduction Analysis (HEC-FDA) software is designed to assist US Army Corps of Engineers (USACE) study team members in using risk analysis methods for flood risk management studies as required by USACE guidance - ER 1105-2-101.
3f Software Planner 2006
Web based enterprise management system with time clock and attendants, issue management, templates and powerful project management tools. The system is modular, you can, aside from the base module, choose any of the following optional modules: Gantt
AMRandom
This aims to supply a Borland Delphi translation of Alan Miller's Random Module for FORTRAN-90. This translation has been done with Dr Miller's approval and is being made FREELY available to all Delphi Developers, though we do ask the Alan Miller and
VI Service Desk
I Service Desk is a complete IT service management solution providing Incident, Service, Knowledge, End User Self Help, and Asset Management. VI Service Desk allows automation of IT processes including Routing, Escalation, Approval, Change Management,Task
BlackMoon Chronicles v.1 30
In the world of Black Moon Chronicles, four separate factions struggle to defend their rank and honor as they seek the approval of their respective leaders, the recognition of their people, and the respect of their enemies.
Norsonic NorBuild v.2 3
The Nor-1028 NorBuild software package is designed to allow the calculation of building acoustic indices in accordance with the Building Regulations Approval Document E.
ElproLOG ANALYZE v.1.01.0100
elproLOG ANALYZE QLS is the software package for qualified systems. This software helps you comply with the provisions of FDA 21 CFR Part 11, GMP, GAMP4, GLP and Annex 11.
FARE Microbial v.1.0
At the request of and in conjunction with FDA, Exponent developed FARE™ Microbial, a modular software for conducting probabilistic microbial risk assessment.
Change Order G701 v.7 8
Fill in the form on-screen: Print or Email the Form with your Data. Only available here. Industry Standard (AIA Format). Professional changes in a consistent format ready for approval and signature.
InkFormulation v.6.0
InkFormulation software provides a fast, accurate and consistent ink formulation, formula creation, storage, approval and retrieval solution for offset, flexographic, gravure and screen-printing inks.